Beautiful Plants For Your Interior

Anodized aluminum is aluminum that has undergone anodizing treatment. Its surface is very durable, corrosion-resistant and aesthetically pleasing. This process involves electrochemical treatment in a series of baths to form an anodic layer directly on the aluminum surface. This layer is an integral part of the aluminum and can prevent problems such as cracking, peeling or flaking. The durability of anodized aluminum is about three times that of untreated aluminum and it is much lighter than stainless steel and copper. The anodizing process forms a tough layer of aluminum oxide that fuses with the metal, greatly enhancing its hardness and strength. This layer is porous, allowing the use of dyes, paints, lubricants and adhesives. This treatment also improves the corrosion resistance and wear resistance of aluminum, enabling it to withstand harsh environmental conditions.

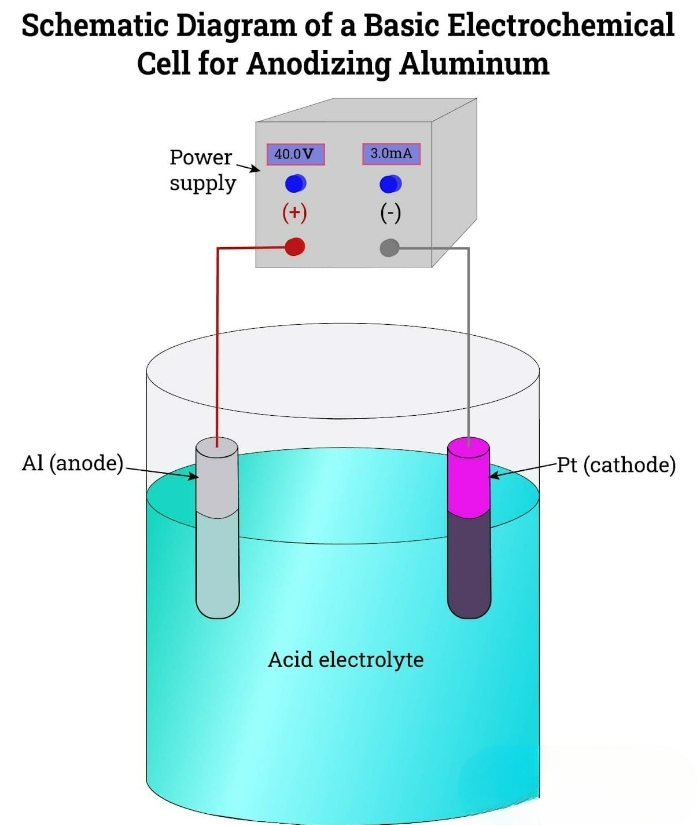
Anodic oxidation forms a robust oxide layer that is fully integrated with the base material, thereby enhancing its hardness and strength. This anodic layer is not prone to cracking, peeling, scratching or flaking because it is chemically bonded to the aluminum rather than a surface coating. The porous structure of this layer enables it to accept dyes, paints, lubricants and adhesives, while providing excellent corrosion and weather resistance, making it suitable for harsh environments. One of the advantages of anodic oxidation is that it does not affect the recyclability of aluminum. Additionally, compared to electroplating and painting methods, this process is considered more environmentally friendly. Anodic oxide components have a smooth, shiny surface, enhancing their aesthetic appeal. Anodic oxidation can also achieve bright and durable coloring, with the resulting film having strong resistance to fading. This makes anodic oxide aluminum a popular choice for decorative and architectural applications. The aluminum anodizing process is an electrolytic passivation technique that can increase the thickness of the natural oxide layer on the aluminum surface. This process forms a solid, stable film, thereby enhancing the inherent corrosion resistance of the aluminum. The anodic oxidation process involves immersing aluminum components in an acidic electrolyte bath and applying direct current (DC) to promote oxidation. During the anodizing process, the aluminum component acts as the anode, while the cathode, typically made of platinum, stainless steel, lead or carbon, completes the circuit. When a voltage is applied, the aluminum component releases positive ions and attracts negative ions, thereby forming an aluminum oxide protective layer on its surface.
Anodizing is typically applied to aluminum, but it can also be used for other non-ferrous metals such as magnesium, zinc and titanium, as well as certain conductive plastics. It is not suitable for carbon steel and other black metals because these materials tend to form iron oxide or rust instead of forming a stable and corrosion-resistant film. Anodizing can be carried out using batch anodizing or continuous anodizing methods. In batch anodizing, items are placed on racks and immersed in a series of baths. After processing, these items will be removed as a group. This method is usually used for cookware, castings and components that have been bent or machined. Continuous anodizing involves unwinding pre-rolled materials that are continuously moved during the anodizing step. Once the process is completed, the materials are rolled up and dispatched. This method is suitable for projects with less deformation, such as wire, sheet, sheet metal and foil.protective layer on its surface.
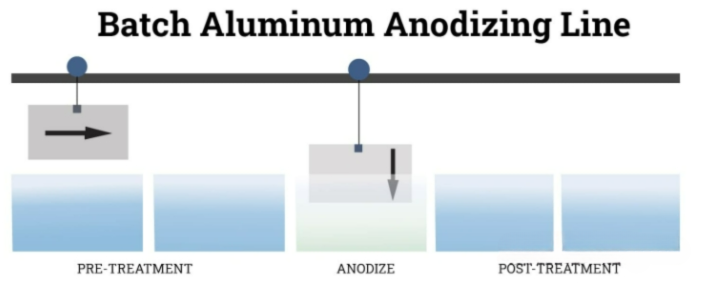
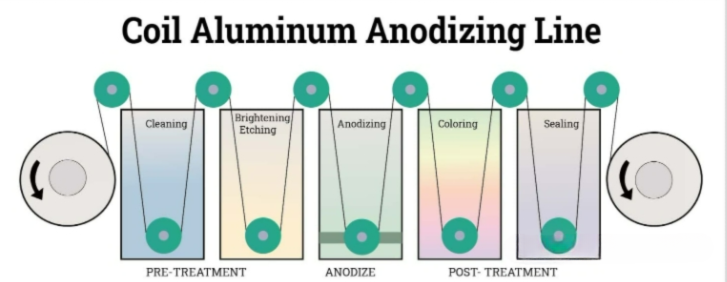
The aluminum anodizing process consists of four key stages:
1、Preprocessing
The pre-treatment stage is indispensable as it significantly affects the final quality and appearance of the anodized surface. This stage involves removing contaminants such as dirt and grease from the original aluminum, which may interfere with the anodizing process, as well as addressing minor surface defects. Additionally, any necessary processing steps, including drilling, cutting, and welding, should be completed before this stage.
Anodic oxidation pretreatment may involve chemical or mechanical methods:
chemical pretreatment
Chemical pretreatment involves using various chemical solutions to clean the aluminum surface. Acid or alkaline cleaners are used to remove dirt and grease, while deoxidizers deal with surface oxides and heat treatment scale. After this, an etching or brightening process is applied to alter the surface texture, thereby creating a unique finish:
- The etching process results in a dull or matte surface. This step removes a uniform layer from the surface of the aluminum parts, thereby reducing minor surface defects. Etching is accomplished by immersing the components in hot sodium hydroxide or trisodium phosphate (alkaline etching) or aqueous ammonium fluoride hydroxide solution (acidic etching).
- The brightening process will produce a glossy or mirror-like effect. In this step, the microscopic peaks and defects on the surface of the aluminum parts are flattened and smoothed. Therefore, the roughness is reduced, resulting in a high-reflective surface. The brightening is achieved by immersing the components in a phosphoric acid or nitric acid bath. The additives are mixed with the acid bath to enhance its whitening ability and reduce toxic fumes.
Mechanical pre-treatment
Mechanical pre-treatment includes techniques such as abrasive polishing, sandblasting and shot peening, which are used to prepare the aluminum surface. Sandblasting and shot peening treatments enhance the fatigue resistance, hardness and adhesion of the coating of the parts. Appropriate coating adhesion is crucial for the durability and effectiveness of the anodized surface.
2、Electrolysis
The anodizing process is centered around electrolysis. At this stage, the aluminum components are immersed in an electrolytic tank filled with positively charged and negatively charged ions. The aluminum components are connected to the positive terminal of the direct current power supply, while the cathode is connected to the negative terminal. When a direct current is applied, as electrons are drawn away from the surface of the aluminum components, they become positively charged. Then these electrons pass through the electrolyte to the cathode, where they react with hydrogen ions to produce hydrogen gas. At the same time, the aluminum cations on the surface react with water to form an oxide layer (Al2O3). The overall chemical reaction involved can be summarized as follows:

During the electrolysis stage, depending on the chemical composition of the electrolyte tank, two different types of oxide films will form:
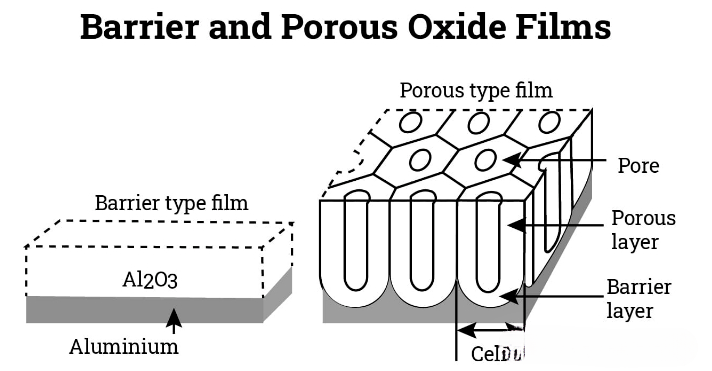
3、Barrier oxide layer
When anodization occurs in a neutral solution (such as ammonium borate, phosphate or tartarate compositions), a barrier oxide film is formed on the surface of the part, in which the aluminum oxide remains insoluble. This type of film is very strong, does not react with the solution, and protects the underlying aluminum from environmental factors. The thickness of the barrier oxide film is affected by the voltage applied between the anode and the cathode. However, the voltage that can be applied before side reactions such as sparking, solute oxidation and oxygen evolution begin is limited.
4、Porous oxide film
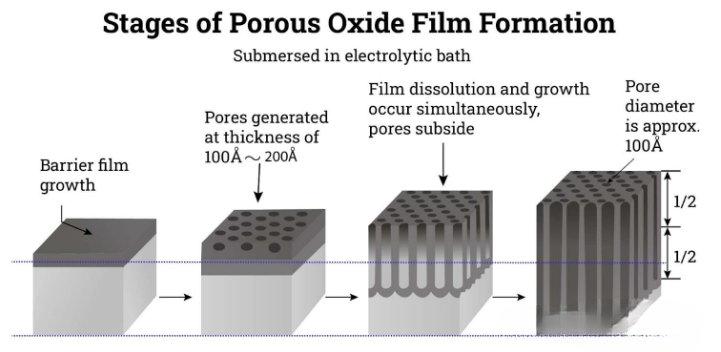
When anodic oxidation occurs in an acidic solution, a porous oxide film forms on the surface, and the acid content is typically around 10%. Sulfuric acid is a commonly used acidic electrolyte, but other options such as phosphoric acid, oxalic acid, chromic acid, and mixtures of inorganic and organic acids have also been adopted. The acidic solution can maintain a high concentration of Al2O3 molecules.
During the anodizing process, the anode reaction leads to the formation of an oxide layer of aluminum oxide. When current passes through the aluminum component, it tends to concentrate in the weaker and more reactive areas on the surface, thereby forming a highly porous or honeycomb structure. The aluminum oxide formed in these pores is dissolved into the acidic bath.
The thickness of this porous oxide film is directly related to the duration of electrolysis and the applied voltage; longer electrolysis time and higher voltage will result in a thicker film with a more distinct columnar structure. Moreover, the size of the pores is also affected by the bath voltage, temperature and acid concentration.
According to the MIL-A-8625 specification for anodic coatings of aluminum and its alloys, there are three main aluminum anodizing processes. Each process endows aluminum with a series of unique properties, tailored to specific applications and requirements.
Type I – Chromic acid anodizing
Type I anodizing uses chromic acid to form an aluminum oxide layer. This method produces a thin oxide film approximately 20-100 microns thick. If properly sealed, it can provide effective corrosion resistance. This film is dielectric and non-conductive, making it suitable as a primer for paint and adhesive applications. Type I anodizing is an ideal choice for components with strict tolerances as it has the least impact on part dimensions.
Parts treated with I-type anodizing have excellent forming properties and can withstand high stress and bending. They are commonly used in aerospace and aircraft applications. Even when dyed black, this film usually appears gray because its thinness limits the absorption of the dye.
Although it has its advantages, type I anodizing can cause environmental problems because chromic acid is toxic and carcinogenic. Factories that carry out type I anodizing treatment must implement specialized wastewater treatment systems to handle the by-products of chromic acid.

Type II – Sulfuric Acid Anodic Oxidation
Type II anodizing is the most popular method of anodizing. It uses sulfuric acid as the electrolyte instead of chromic acid. This process produces a porous oxide layer that can effectively absorb dyes, paints, and adhesives, making it an ideal choice for decorative applications. The oxide film produced by Type II anodizing is thicker, ranging from 100 to 1000 microns, and retains dielectric and non-conductive properties.
Compared with I-type anodized parts, the parts treated with II-type anodization exhibit superior wear resistance and corrosion resistance, and are usually harder. These characteristics make II-type anodized components suitable for a wide range of applications, including decorative items, architectural elements, consumer electronics, military kitchenware, weapons, and optical components.
Type II anodizing is also more cost-effective than Type I anodizing because it has lower chemical costs, lower energy consumption, and a simpler waste treatment process.

Type III – Hard Anodizing
Type III anodizing is similar to Type II, using sulfuric acid as the electrolyte. However, it can operate under more intense conditions, including higher current density, higher voltage, and lower temperature. This process produces a significantly thicker and porous oxide film, with a thickness exceeding 1000 microns. The resulting coating is very hard and durable. Nevertheless, Type III anodizing may not be suitable for components with very strict tolerances due to minute size variations. The obtained film is usually dark in color and can be left uncolored or dyed black.
Type III is known as hard-coated anodizing, which possesses excellent wear resistance and durability as well as good electrical insulation properties. The addition of PTFE can further reduce the friction coefficient, making it suitable for components subjected to frequent frictional stress. Although it performs well in terms of corrosion resistance, the increased oxide thickness can affect the fatigue resistance of the parts.
Type III anodized components are typically used in demanding fields such as military and aerospace. Applications include sliding parts, linear guides, pistons, valves, hinges, gears, insulating plates, and compressor accessories.
Although type III anodizing is as environmentally friendly as type II anodizing, its cost is higher due to the strict process requirements it demands.

Other types of aluminum anodizing processes include:
Boron sulfate anodizing
Borosulfuric acid anodizing (BSAA) is an alternative to Type I anodizing, addressing the environmental and safety issues associated with the Type I process. BSAA offers similar benefits in terms of adhesion to paints, lubricants, and adhesives, and has excellent corrosion resistance. It is suitable for parts requiring strict tolerances and is commonly used in the aerospace and aircraft industries.
phosphoric acid anodizing
Phosphoric acid anodizing (PAA), also known as the Boeing process, is another alternative to Type I anodizing. It uses phosphoric acid to produce an oxide film. The films made by PAA have a unique rough texture, featuring protrusions and whiskers, which enhance their adhesion properties. These films also have good resistance to high humidity, making PAA suitable for preparing aluminum surfaces for use in adhesive primer applications. PAA is often used in structural adhesive bonding processes.
Thin-film sulfuric acid anodic oxidation (Type IIb)
Tensile Film Sulfuric Acid Anodic Oxidation (TFSAA) employs a sulfuric acid-based electrolyte solution based on Type II anodic oxidation, with a lower concentration. This results in an oxide film that is thinner than those produced by Type II and Type III anodic oxidation, making TFSAA a substitute for Type I anodic oxidation.
Due to the thinness of the oxide film, the parts treated by TFSAA have higher fatigue strength, making them highly suitable for high-stress applications. Moreover, these components are easy to be dyed. However, their corrosion resistance is not as strong as that of type II and type III anodized components.
Transparent anodizing
Transparent anodizing begins with sulfuric acid anodizing and ends with the sealing of the component in a hot water bath. This method produces a colorless finish, forming a uniform and transparent layer on the aluminum surface, thereby enhancing its visual appeal. Usually, the anodized material is not colored, and its color varies according to the thickness of the oxide layer. Transparent anodizing is commonly applied to automotive decorations, window frames, railings, wall panels, photographic plates, and extruded profiles.

Bright Dip Anodizing
The bright immersion anodizing process involves the pre-treatment with a mixture of phosphoric acid and sulfuric acid to obtain a shiny and highly reflective surface. After the pre-treatment, type II anodizing is carried out. Then the anodized part is immersed in the coloring dye and sealed with a porous film. The final appearance depends on the grade of the aluminum alloy. Nevertheless, the bright immersion anodizing enhances the overall aesthetic appeal of the parts.
Black anodized finish
Black anodizing begins with the typical anodizing process, and then organic or inorganic black dyes are applied to the anodized surface. These dyes are specifically designed to color aluminum components. Compared to organic dyes, inorganic dyes (such as ammonium ferric oxalate) have excellent light resistance, meaning they are less prone to fading under light exposure. Alternatively, black anodized finishes can be achieved through the electro-deposition of colored metals.
Color anodizing
Colored anodizing involves the standard anodizing process followed by immersion coloring with organic dyes. The available organic dyes offer a wider range of colors compared to inorganic dyes. Colored anodizing is mainly used for aesthetic applications. However, the light resistance produced by organic dyes is not as good as that of black anodized components.
Put colour on
During the coloring stage, the dyes or pigments penetrate into the porous alumina layer formed through electrolysis. The dyes bind to this oxide film. The “coating” of the anodic alumina is durable and scratch-resistant. The high porosity of the surface makes it highly suitable for the absorption of dyes and pigments.
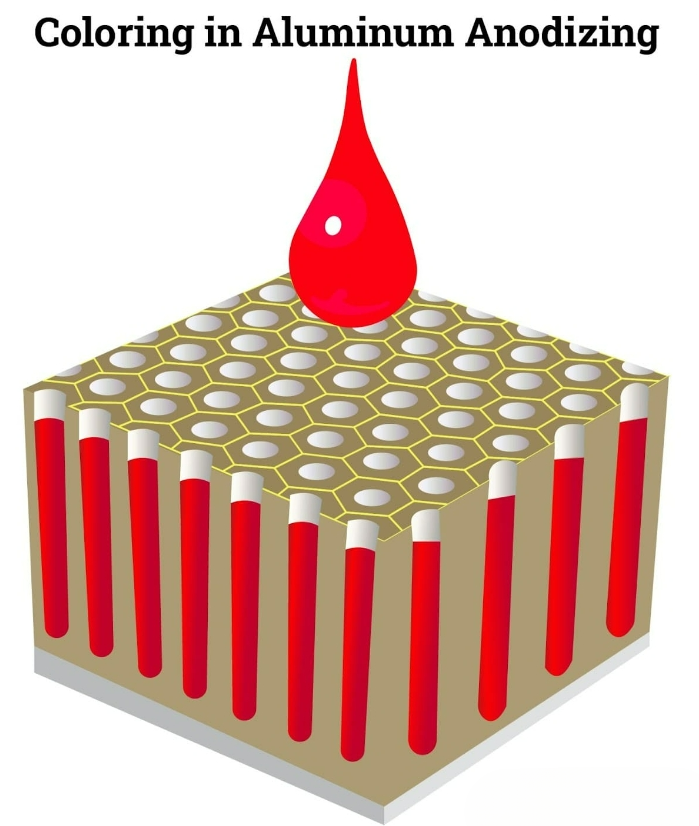
Once the anodic oxidation solution has been thoroughly rinsed off and the parts have dried, the coloring process begins. This can be achieved through various methods:
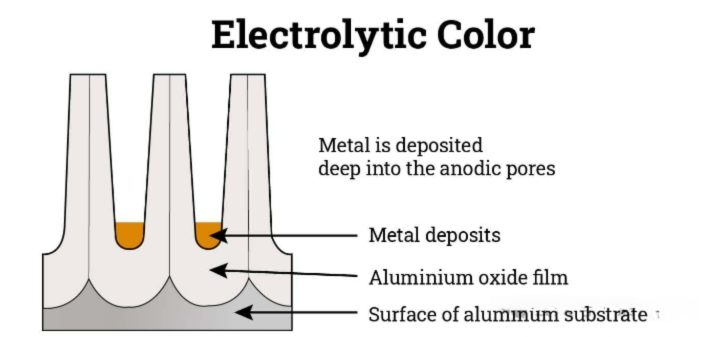
Two-step anodic oxidation by electrolysis
In the two-step anodic oxidation process by electrolysis, the component is first subjected to anodic oxidation treatment through electrolysis, and then immersed in a bath containing metal salts. Subsequently, an electric current is applied to cause the metal ions to deposit in the pores of the oxide layer. This deposition gives the anodic oxidation surface a unique color. Commonly used coloring metals include cobalt, tin, copper, and nickel. The final color and quality depend on the type of metal used and the concentration of the metal deposits in the pores.
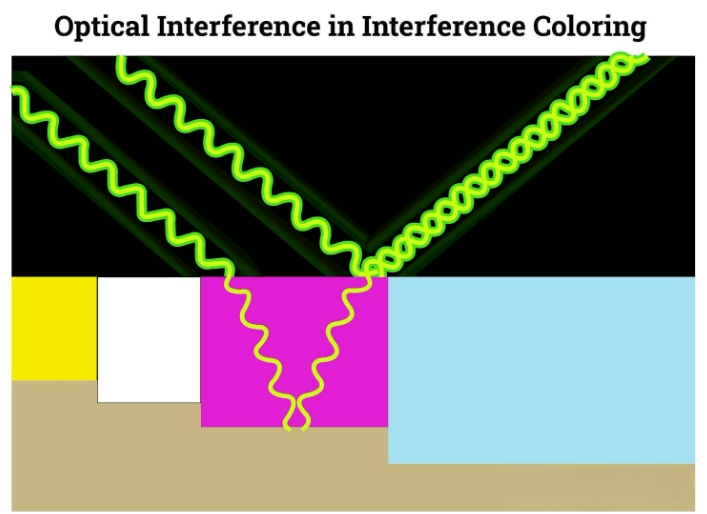
Interferometric coloration
Interference coloring involves widening the bottom of the oxide film pores to allow for the deposition of more metal ions through electrolysis. This technique produces light-resistant colors such as blue, green, yellow and red, which are generated through the optical interference of visible light waves.
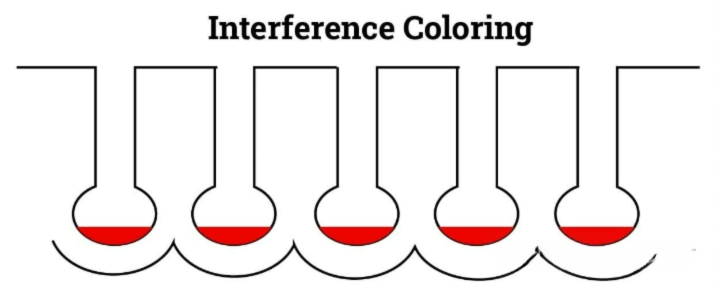
Overall coloring
Overall coloring combines the anodizing and coloring processes into one step, coloring the oxide film during the anodizing process. This technique uses a bath containing organic acids and sulfuric acid to produce a thicker and more wear-resistant coating. However, this is an expensive method, and producing colored oxide films may be more challenging. The achievable color range is usually limited to light yellow to dark yellow, bronze, brown, black, and gray shades.

Dyeing by immersion
In the dip coloring process, the anodic oxidation components are immersed in a bath containing dyes. The dyes adhere to the pore surfaces of the oxide film. The final color obtained is influenced by the specific dyes used and their chemical properties. This method is cost-effective and can apply various colors to aluminum parts. However, compared to the films produced by other technologies, the resulting colored films have poorer resistance to ultraviolet rays.
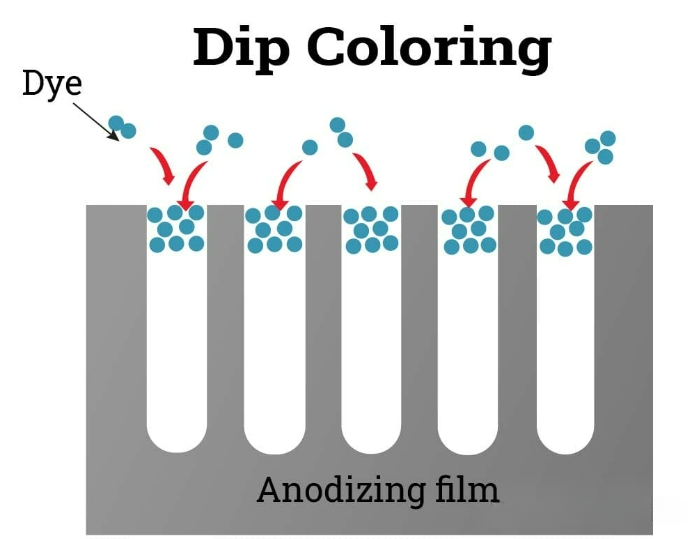
In some processes, lubricants or adhesives are used instead of colorants. For parts that do not require coloring, the coloring step is omitted.
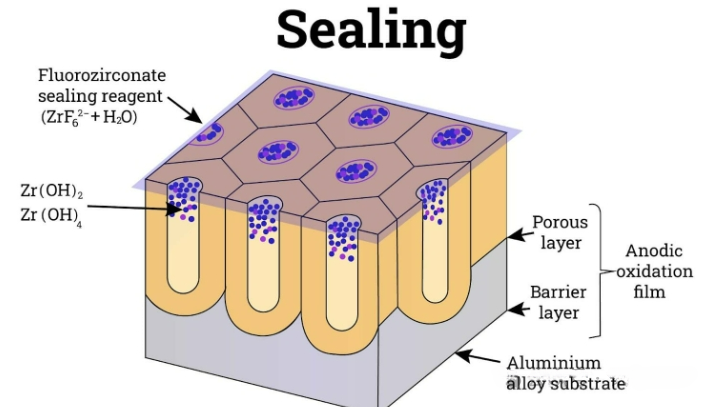
seal
The final step of the anodizing process is sealing, which fixes the absorbed dyes, lubricants or adhesives within the porous oxide layer. This sealing process protects the porous film from corrosion, staining and unwanted substances absorption, and also prevents color fading. Sealing is achieved by using a sealant, which can close the pores or reduce their diameter. Due to the sensitivity of the oxide film, sealing must be carried out immediately after coloring.